By photo-exciting a metal surface utilizing modern femtosecond
(10^-15 s) laser technology one can bring the metal electronic and
phononic subsystem out of thermal equilibrium. This is because the
light is absorbed by the electrons, which hence have a high
temperature distribution (T_el) and chill down only slowly by coupling to
lattice phonons (T_ph). This non-equilibrium, which persists only for a few
picoseconds (10^-12 s), can be used to examine the nature of a
chemical reaction taking place on the surface. It can moreover be
used to drive reactions that can not be initiated by simply
heating the surface.
Here you find a visualization of the oxidation of carbon monoxide
(CO) with coadsorbed oxygen (O) on the (001)-face of a ruthenium
(Ru) single crystal.
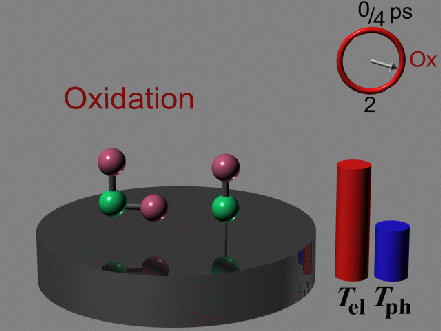
The CO oxidation is initiated by the activation of a strongly
bound oxygen atom through vibrational heating. This heating is
provided by interaction of the oxygen and the hot metal electrons
only during the short span of time of thermal nonequilibrium in the
metal. Since the oxygen activation is the rate-determining step,
the reaction with the CO occurs to form carbon dioxide, which subsequently desorbs.
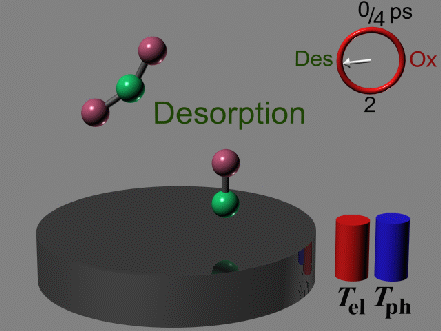
The competing reaction, the simple desorption of CO, is driven
again by vibrational heating, which in this case is provided by
the lattice phonons (there is only weak interaction between the
metal electrons and the CO). Therefore the desorption occurs
at times, when the phonons are hot: after equilibrium with the
electrons is reached.
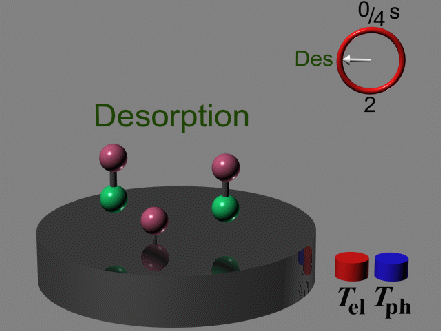
It is the separation of timescales, with the oxidation occurring
prior to the desorption, which enables the oxidation at all. Therefore
the oxidation can only take place, if the electronic and phononic system
are out of equilibrium, which is only under femtosecond laser excitation conditions.
By thermal heating of the surface, where the electrons and phonons
are always in equilibrium, the desorption is the only pathway for the
reaction, because much less heat is needed to desorb the weakly
bound CO than to activate the strongly bound O to oxidize the CO.
Once the oxygen is excited thermally there is no more CO left on the
surface to react with.
(Reference: M. Bonn, S. Funk, Ch. Hess, D. N. Denzler, C. Stampfl,
M. Scheffler, M. Wolf and G. Ertl in
Phonon- versus electron-mediated desorption and oxidation of CO on Ru(0001).
Science 285, pp. 1042-1045, 13. Aug. 1999)